
Environment and energy
Under this general header, a detailed and structured point of view regarding modern way of living is implied. The interaction of the mankind with the urban and natural environment is considered as of major importance for the next decades therefore GAP energy Ltd, is focusing at the integration of modern ways of power production and power storage.
GAP energy as a General Contractor has been involved in many projects regarding the renewable energy like Photovoltaic parks and Wind Generators farms.
In front of the new challenges of incorporating new and advanced technologies at the production and storage of the renewable energy, Gap Energy has established a separate division especially dedicated in the Hydrogen and Fuel cells technologies.
Hydrogen and Fuel cells
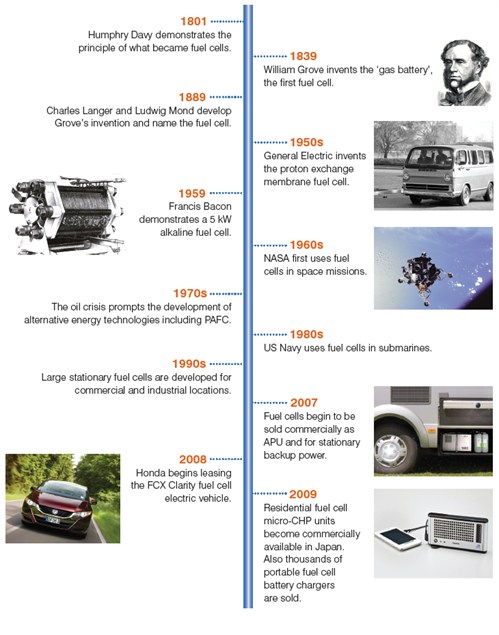
Introduction
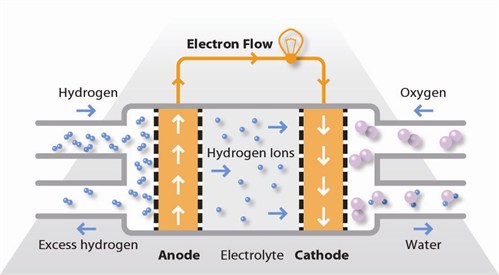
Fuel cells generate electricity by an electrochemical reaction in which oxygen and a hydrogen-rich fuel combine to form water. Unlike internal combustion engines, the fuel is not combusted, the energy instead being released electro- catalytically. This allows fuel cells to be highly energy efficient, especially if the heat produced by the reaction is also harnessed for space heating, hot water or to drive refrigeration cycles.
A fuel cell is like a battery in that it generates electricity from an electrochemical reaction. Both batteries and fuel cells convert chemical potential energy into electrical energy and also, as a by-product of this process, into heat energy. However, a battery holds a closed store of energy within it and once this is depleted the battery must be discarded, or recharged by using an external supply of electricity to drive the electrochemical reaction in the reverse direction. A fuel cell, on the other hand, uses an external supply of chemical energy and can run indefinitely, as long as it is supplied with a source of hydrogen and a source of oxygen (usually air).
There are several different types of fuel cell but they are all based around a central design. A fuel cell unit consists of a stack, which is composed of a number of individual cells. Each cell within the stack has two electrodes, one positive and one negative, called the cathode and the anode. The reactions that produce electricity take place at the electrodes. Every fuel cell also has either a solid or a liquid electrolyte, which carries ions from one electrode to the other, and a catalyst, which accelerates the reactions at the electrodes. The electrolyte plays a key role – it must permit only the appropriate ions to pass between the electrodes. If free electrons or other substances travel through the electrolyte, they disrupt the chemical reaction and lower the efficiency of the cell.
Fuel cells are generally classified according to the nature of the electrolyte (except for direct methanol fuel cells which are named for their ability to use methanol as a fuel), each type requiring particular materials and fuel. Each fuel cell type also has its own operational characteristics, offering advantages to particular applications. This makes fuel cells a very versatile technology.
As a result, fuel cells have a broader range of application than any other currently available power source – from toys to large power plants, from vehicles to mobile chargers, and from household power to battlefield power.
Technologies
A fuel cell is like a battery in that it generates electricity from an electrochemical reaction. Both batteries and fuel cells convert chemical potential energy into electrical energy and also, as a by-product of this process, into heat energy. However, a battery holds a closed store of energy within it and once this is depleted the battery must be discarded, or recharged by using an external supply of electricity to drive the electrochemical reaction in the reverse direction. A fuel cell, on the other hand, uses an external supply of chemical energy and can run indefinitely, as long as it is supplied with a source of hydrogen and a source of oxygen (usually air). The source of hydrogen is generally referred to as the fuel and this gives the fuel cell its name, although there is no combustion involved. Oxidation of the hydrogen instead takes place electrochemically in a very efficient way. During oxidation, hydrogen atoms react with oxygen atoms to form water; in the process electrons are released and flow through an external circuit as an electric current. Fuel cells can vary from tiny devices producing only a few watts of electricity, right up to large power plants producing megawatts. All fuel cells are based around a central design using two electrodes separated by a solid or liquid electrolyte that carries electrically charged particles between them. A catalyst is often used to speed up the reactions at the electrodes. Fuel cell types are generally classified according to the nature of the electrolyte they use. Each type requires particular materials and fuels and is suitable for different applications. –
Applications
The use of fuel cells is categorized into three broad areas: portable power generation, stationary power generation, and power for transportation. A category for fuel and infrastructure is included, relating to the production, distribution, storage and dispensing of fuels for fuel cells, as this is crucial to implementing fuel cell technology.
Transport
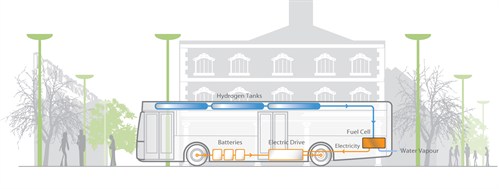
Fuel cells for transport as defined as any units that provide propulsive power to a vehicle, directly or indirectly (i.e. as range extenders). This includes the following applications for the technology: Forklift trucks and other goods handling vehicles such as airport baggage trucks etc Two- and three-wheeler vehicles such as scooters Light duty vehicles (LDVs), such as cars and vans Buses and trucks Trains and trams Ferries and smaller boats Manned light aircraft Unmanned aerial vehicles (UAVs) and unmanned undersea vehicles (UUVs), for example, for reconnaissance Fuel cell LDVs have so far seen limited use but this is set to change as most major automakers have targeted 2015 for initial commercial sales of their fuel cell vehicles. Initial locations for this rollout will most likely concentrate around clusters of early hydrogen refueling infrastructure in Japan, Germany and the USA, and will then spread outwards from these centers as the market is established. The fuel cell bus sector is showing year-on-year growth, with more prototypes being unveiled. Successful deployments have taken place in Europe, Japan, Canada and the USA but the high capital cost is still a barrier to widespread adoption. However, it is hoped that soon after 2014 fuel cell bus prices will be comparable to diesel-hybrid bus prices. ‘Niche’ transport consists of a number of sub-applications with differing levels of commercial success to date. Materials handling vehicles account for over 90% of niche transport shipments, with PEMFC technology dominating. This market has seen much success in the USA so far. Unmanned aerial vehicles (UAVs), e-bikes and trains amongst others are still under development with limited deployments to date –
Portable
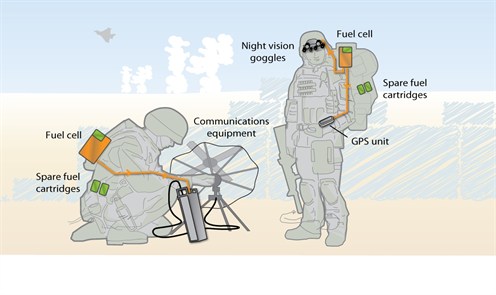
Portable fuel cells are defined as those which are built into, or charge up, products that are designed to be moved. These include military applications (portable soldier power, skid mounted fuel cell generators etc), Auxiliary Power Units (APU) (e.g. for the leisure and trucking industries), portable products (torches, vine trimmers etc), small personal electronics (mp3 players, cameras etc), large personal electronics (laptops, printers, radios etc), education kits and toys. To power this range of products, portable fuel cells are being developed in a wide range of sizes ranging from less than 5 W up to 500 kW. Fuel cells are being sold commercially for these applications, APU, small personal electronics, education kits and toys (these we classify as micro fuel cells). A micro fuel cell is defined as a unit with a power output of less than 5 W. The difference between small and large personal electronics is that the smaller devices, such as cameras or mobile phones only draw around 3 W of power, whereas a laptop can use up to 25 W, requiring a fuel cell of higher power density. APU systems comprise the largest MW share of this sector, with very successful deployments of DMFC systems throughout the European leisure sector. Portable fuel cells typically replace or augment battery technology and exploit either PEM or DMFC technology. PEM fuel cells use direct hydrogen, with no point-of-use emissions, whereas DMFCs emit small quantities of CO2. The main drivers for fuel cells in portable applications are as follows: off-grid operation longer run-times compared with batteries rapid recharging significant weight reduction potential (for soldier-borne military power) convenience, reliability, and lower operating costs also apply –
Stationary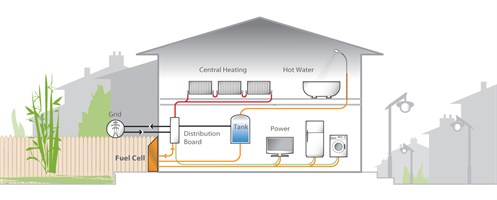
Stationary fuel cells are defined as units which provide electricity (and sometimes heat) but are not designed to be moved. These include combined heat and power (CHP), uninterruptible power systems (UPS) and primary power units. CHP units are sized between 0.5 kWe and 10 kWe, use either PEM or SOFC technology and take advantage of the fact fuel cells generate heat alongside electricity. By making use of this heat, for example to make hot water, the overall efficiency of the system increases. Fuel cells are also more efficient at generating electricity which gives CHP units overall efficiencies of 80-95%. Residential CHP units have been deployed extensively in Japan with more than 10,000 cumulative units by the end of 2010 providing home power and heating. South Korea has also deployed CHP units for residential use but, as in Japan, their purchase still relies upon government subsidies. UPS systems provide a guaranteed supply of power in the event of grid interruption; this market can be divided into five sub-sectors: Off-line short run-time systems for telecommunication base stations; Off-line extended run-time systems for critical communication base stations such as Terrestrial Trunked Radio (Tetra) networks; Off-line extended run-time rack mountable systems for data centers; On-line rack mountable systems for data centers; Off-line systems for residential use. Of these five sectors the first three are the most advanced and comprise the majority of shipments to date. Fuel choice varies by region with natural gas and LPG dominating in Asia, hydrogen prevalent in the USA, and in Europe some adopters are trialing methanol. Large stationary refers to multi-megawatt units providing primary power. These units are being developed to replace the grid, for areas where there is little or no grid infrastructure, and can also be used to provide grid expansion nodes. Four technology types serve the large stationary market: SOFC, MCFC, PEMFC and PAFC. The manufacturing of these units is predominately located in the USA and Japan.
Fuel and Infrastructure
Infrastructure
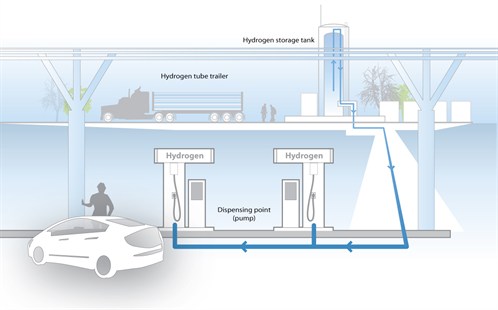
Infrastructure relates to the equipment and systems needed to produce, distribute, store, monitor and dispense fuel, specifically hydrogen, for fuel cells. Hydrogen as a Fuel Most types of fuel cells require hydrogen as a fuel source. The long-term goal of fuel cell research is to produce a totally non-polluting power source. In order to achieve this, the fuel cell must run on hydrogen generated by renewable means. The technology to do this does exist (see below), but the infrastructure to achieve this efficiently and cheaply is still under development. In the meantime, fuel cells will be powered by hydrogen extracted from fossil fuels (oil and natural gas) by reforming. Reforming can either take place on a very large scale at source, or locally at the point of use by small reformers integrated with the fuel cell. More detail on this is given below. Producing Hydrogen by Electrolysis Hydrogen can be produced by the electrolysis of water: splitting of water into its component elements. This process takes place in an electrolyser, which can be described as a ‘reverse’ fuel cell: instead of combining hydrogen and oxygen electrochemically to produce electricity and water as a fuel cell does, an electrolyser uses an electrical current and water to produce hydrogen and oxygen. The key issue here is the source of the electrical current. If grid electricity is used, the hydrogen has a carbon footprint associated with it due to the coal or gas that must be burnt to produce the necessary electricity. However, if the electricity is obtained from renewable energy such as wind or solar power, the hydrogen can be produced in a completely carbon-free way. Electrolysers exist and many commercial versions of various capacities are available on the market. A number of companies have called for these to be used in combination with wind or solar power to produce hydrogen for fuel cells. Producing Hydrogen by Fuel Reforming Hydrogen can be generated by reforming of hydrocarbon fuels such as natural gas, methanol, gasoline or ethanol. These are not necessarily fossil fuels; reforming of bio-ethanol, for instance, is equally possible and this would then also be a source of renewable hydrogen. Generally, there are two different kinds of reforming: external reforming, which is carried out before the fuel reaches the fuel cell itself, and internal reforming, which takes place within the fuel cell stack. External reforming could be carried out at a refinery or chemical plant and the hydrogen delivered by pipeline to filling stations. External reforming can also take place in a reformer integrated with the fuel cell, so that the fuel cell system can be fed a hydrocarbon fuel (town gas for instance). The reformer then extracts the hydrogen from the fuel and feeds it to the fuel cell. In this instance, there will still be emissions from the system at point of use, but due to the higher efficiency of fuel cells these will be less than if the gas were simply combusted. Reforming Technologies Steam Reforming Fuel is mixed with steam in the presence of a base metal catalyst to produce hydrogen and carbon monoxide. This method is the most well-developed and cost-effective for generating hydrogen and is also the most efficient, giving conversion rates of 70% to 80% on a large scale. Partial Oxidation Reforming Partial oxidation can be used for converting methane and higher hydrocarbons but is rarely used for alcohols. This method involves the reaction of the hydrocarbon with oxygen to liberate hydrogen, and produces less hydrogen for the same amount of fuel than steam reforming. The reaction is, however, exothermic and therefore generates heat. This means that the reaction can be initiated by a simple combustion process leading to quick start-up. Once the system is running it then requires little external heating to keep going. The technology is preferred where there is little access to natural gas or an abundance of oil. Auto-thermal reforming combines the endothermic steam reforming process with the exothermic partial oxidation reaction, therefore balancing heat flow into and out of the reactor. These systems can be very productive, fast-starting and compact, and have been demonstrated with methanol, gasoline and natural gas. A number of auto and oil companies are also working on proprietary versions of this technology. Hydrogen Storage Hydrogen is the lightest chemical element and offers the best energy to weight ratio of any fuel. The major drawback to using hydrogen is that it has the lowest storage density of all fuels. However, it is possible to store large quantities of hydrogen in its pure form by compressing it to very high pressure and storing it in containers which are designed and certified to withstand the pressures involved. In this way it can either be stored as a gas, or cooled to below its critical point and stored as a liquid. Hydrogen can also be stored in solid form, in chemical combination with other elements (there are a number of metals which can ‘absorb’ many times their own weight in hydrogen). The hydrogen is released from these compounds by heating or the addition of water. Other storage mediums are being investigated, for example carbon nanotubes and glass microspheres. Other Fuels For high temperature systems such as molten carbonate and solid oxide cells, it is possible to supply a hydrocarbon (e.g. natural gas or methanol) directly to the fuel cell without prior reforming. The high temperature allows the reforming stage to take place within the fuel cell structure. In practice, some preliminary reforming or purifying of the fuel is often carried out. The exception to this is direct methanol fuel cells, in which a catalyst on the anode draws the hydrogen from liquid methanol, eliminating the need for a fuel reformer. Therefore, as the name suggests, pure methanol can be used as fuel –
Benefits
Fuel cells have various advantages compared to conventional power sources, such as internal combustion engines or batteries. Although some of the fuel cells’ attributes are only valid for some applications, most advantages are more general. Benefits include: Fuel cells have a higher efficiency than diesel or gas engines. Most fuel cells operate silently, compared to internal combustion engines. They are therefore ideally suited for use within buildings such as hospitals. Fuel cells can eliminate pollution caused by burning fossil fuels; for hydrogen fuelled fuel cells, the only by-product at point of use is water. If the hydrogen comes from the electrolysis of water driven by renewable energy, then using fuel cells eliminates greenhouse gases over the whole cycle. Fuel cells do not need conventional fuels such as oil or gas and can therefore reduce economic dependence on oil producing countries, creating greater energy security for the user nation. Since hydrogen can be produced anywhere where there is water and a source of power, generation of fuel can be distributed and does not have to be grid-dependent. The use of stationary fuel cells to generate power at the point of use allows for a decentralized power grid that is potentially more stable. Low temperature fuel cells (PEMFC, DMFC) have low heat transmission which makes them ideal for military applications. Higher temperature fuel cells produce high-grade process heat along with electricity and are well suited to cogeneration applications (such as combined heat and power for residential use). Operating times are much longer than with batteries, since doubling the operating time needs only doubling the amount of fuel and not the doubling of the capacity of the unit itself. Unlike batteries, fuel cells have no “memory effect” when they are getting refueled. The maintenance of fuel cells is simple since there are few moving parts in the system.
(Source: Fuel Cell Today)


 GAP ENERGY is a general contracting and trading company, specialized in complete industrial projects and trading of commodities in the Oil and Gas Sector.
GAP ENERGY is a general contracting and trading company, specialized in complete industrial projects and trading of commodities in the Oil and Gas Sector.
